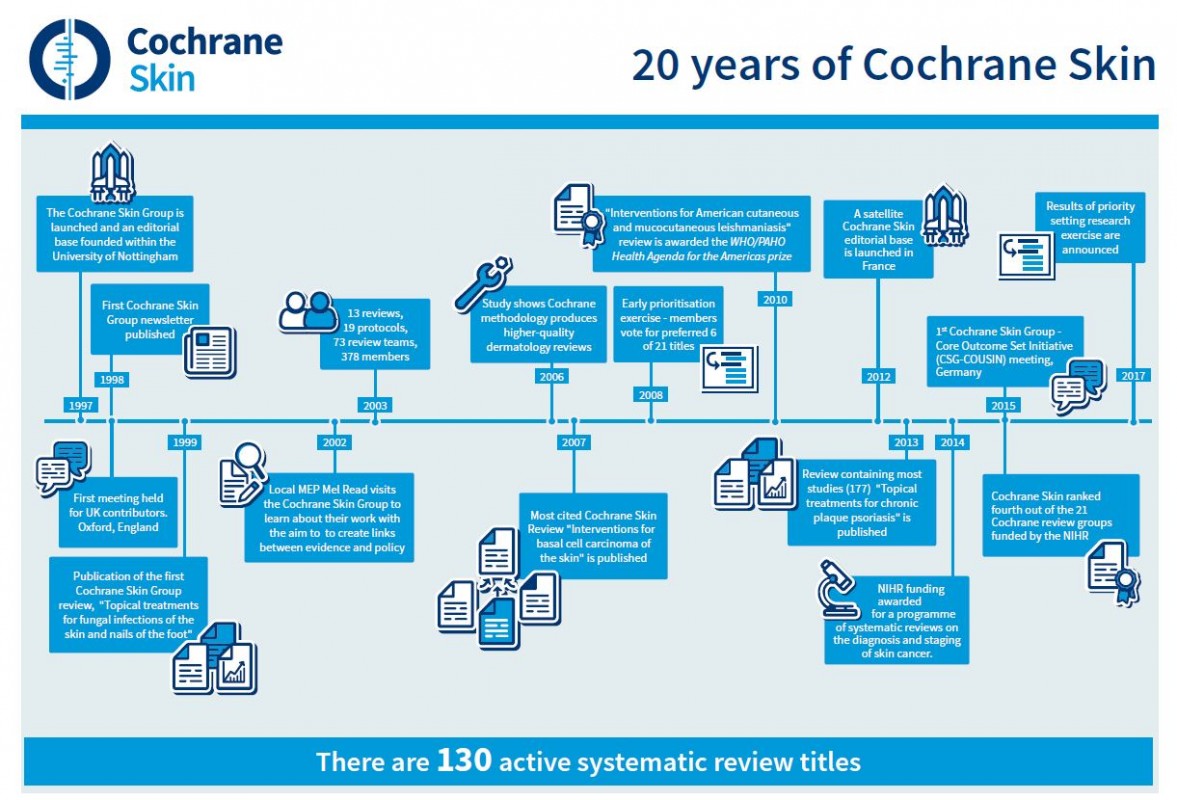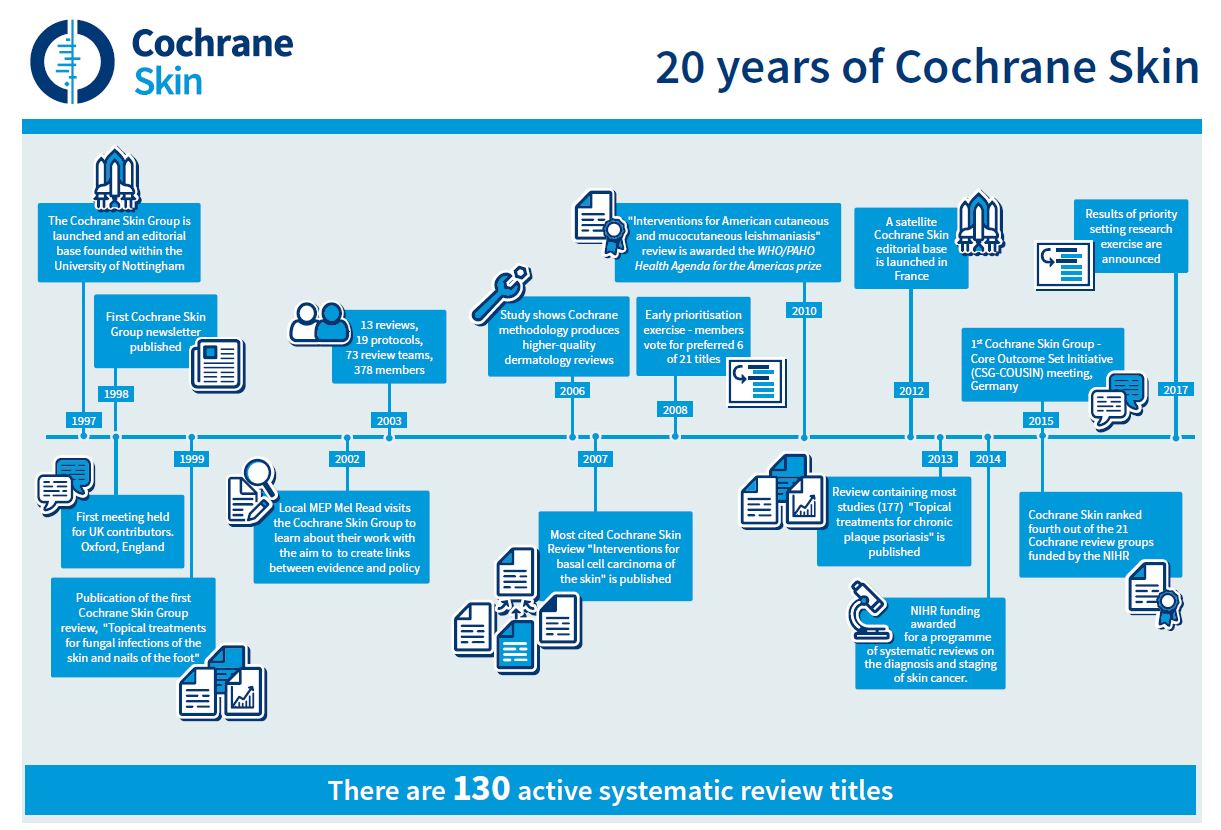
World-leading dermatologists and their patients are celebrating huge improvements in the treatment of skin diseases like skin cancer, psoriasis, and acne as the Cochrane Skin Group marks its 20th anniversary this week. The effects of the 120 plus published Cochrane Skin Reviews have been far-reaching and have had real impacts on patient care. These include skin cancer, skin allergies, and acne, blistering diseases, hair disorders like alopecia, fungal infections and psoriasis as well as tropical parasitic diseases.
The group is coordinated by the University of Nottingham’s Centre of Evidence Based Dermatology and is part of the global Cochrane network that carries out systematic reviews of primary research covering all areas of health and health policy. Cochrane is internationally recognised as setting the highest standards in evidence-based health care resources.
The effects of the 120 plus published Cochrane Skin Reviews have been far-reaching and have had real impacts on patient care. These include skin cancer, skin allergies and acne, blistering diseases, hair disorders like alopecia, fungal infections and psoriasis as well as tropical parasitic diseases.
“By systematically reviewing and summarising all relevant clinical trials, Cochrane Skin tries to drive up the quality of primary research into skin disease prevention and treatments,” says Professor Hywel Williams, the visionary founder of Cochrane Skin. “A lot of dermatology clinical trials have addressed unimportant questions that mean little to patients, and which have been designed and reported badly. By sorting out the wheat from the chaff, Cochrane Skin draws a line in the sand in order to stimulate better primary research.”
Inspired by a call to action from one of the original Cochrane founders, Sir Iain Chalmers, in the British Medical Journal in 1992, Professor Williams set about building Cochrane Skin, which was officially registered with Cochrane (originally The Cochrane Collaboration) on 12th September 1997.
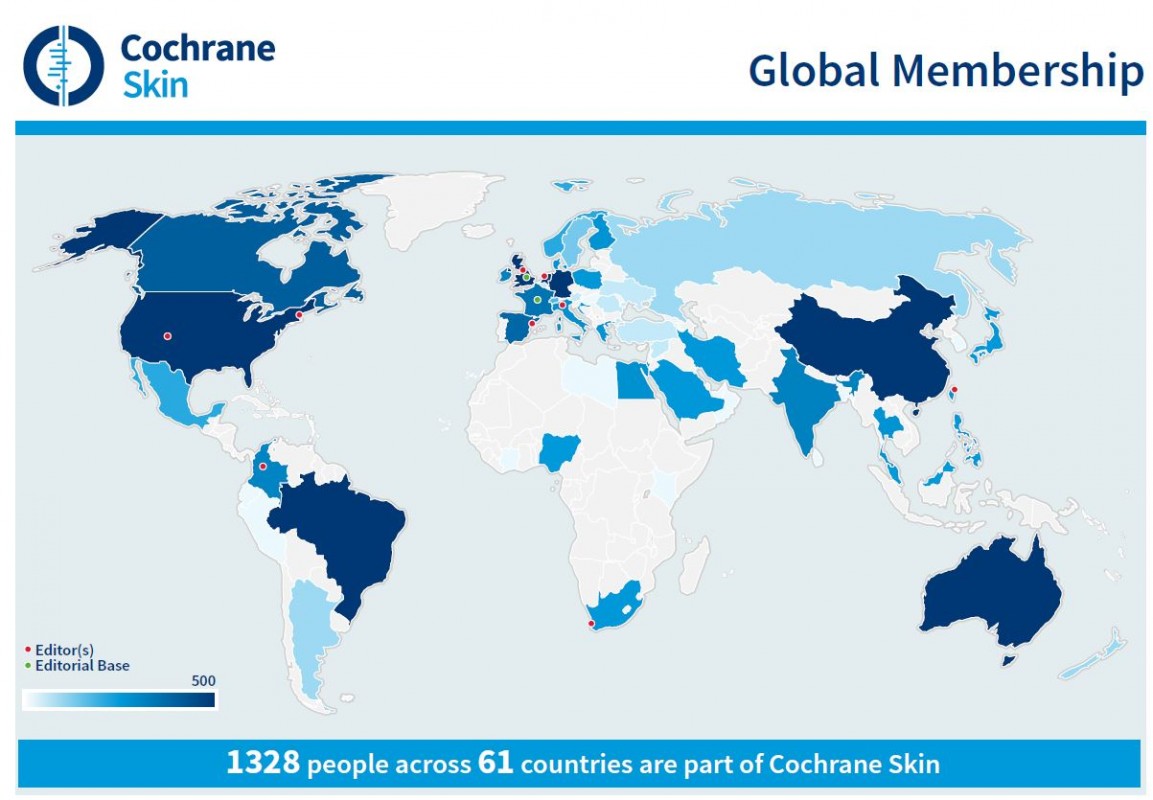
At present more than 1,300 people from 61 different countries contribute to the work of the Cochrane group, with an established Satellite group in France and others planned in South America and the USA.
From the outset, Cochrane Skin has closely involved patient groups in its work. Maxine Whitton MBE, a co-founder of The Vitiligo Society, was formerly Chairman and is now a Patron, has had the skin pigmentation condition ‘vitiligo’ since childhood. Maxine said:
“Professor Williams encouraged and supported me in producing the first review of interventions for vitiligo. This review retrieved just 19 randomised trials, mostly of poor quality, published since 1966, highlighting the need for more research and well-designed trials as the basis for informed decisions on treatments. We conducted two further Cochrane Skin reviews published in 2010 (57 trials) and 2015 (96 trials).
“The review has stimulated further vitiligo research and projects. For example, the Vitiligo Society has helped us in the recruitment of participants for a pilot study of psychological interventions for vitiligo and also a large multi-centre trial combining topical treatment and NB-UVB for vitiligo (Hi-Light) based at Nottingham, which is ongoing.”
Cochrane Skin will be celebrating its 20th anniversary as the first Global Evidence Summit (GES) takes place in Cape Town, South Africa (13th – 16th September 2017). This event sees Cochrane join with four other leading organisations – the Guidelines International Network, The Campbell Collaboration, the International Society for Evidence-based Health Care, and the Joanna Briggs Institute – to focus on the opportunities and challenges facing low- and middle-income countries. The theme of the GES, ‘Using Evidence. Improving Lives’, will highlight and promote evidence-informed approaches to health policy and development, offering the most cost-effective interventions.

Cochrane Skin is grant-funded by the National Insitute for Health Research (NIHR) Systematic Reviews Programme.
- Visit the Cochrane Skin website
- Read the University of Nottingha blog post
- More information is available from Professor Hywel Williams, Centre of Evidence Based Dermatology, Faculty of Medicine and Health Sciences on +44 (0)115 or email hywel.williams@nottingham.ac.uk or Emma Rayner in the Communications Office at The University of Nottingham, on +44 (0)115 951 5793, emma.rayner@nottingham.ac.uk