Why does screening matter?
Screening aims to identify a condition in people who may not be showing any symptoms. Some people may have the COVID-19 virus but appear healthy or have only mild symptoms. It is important to identify infected people so they can stay away from others and seek appropriate care. Incorrectly identifying COVID-19 in healthy people could lead to unnecessary self-isolation and further tests. Incorrectly identifying no infection in infected people could spread the virus.
Screening for COVID-19 can include temperature checks, or asking about international travel or contact with COVID-19 cases, or rapid tests. Screening can occur over the telephone, online, or in person, in homes, clinics, workplaces, airports or schools.
What did the review study?
We wanted to identify:
· the benefits and negative effects of screening apparently healthy people for COVID-19 infection;
· whether screening can identify those with and without the virus correctly.
To answer these questions rapidly, we shortened some steps of the normal Cochrane Review process. We are confident these changes do not affect our overall conclusions.
What did we do?
We looked for studies that screened people who had not sought care for potential COVID-19 symptoms.
This review includes evidence up to May 2020.
Key results
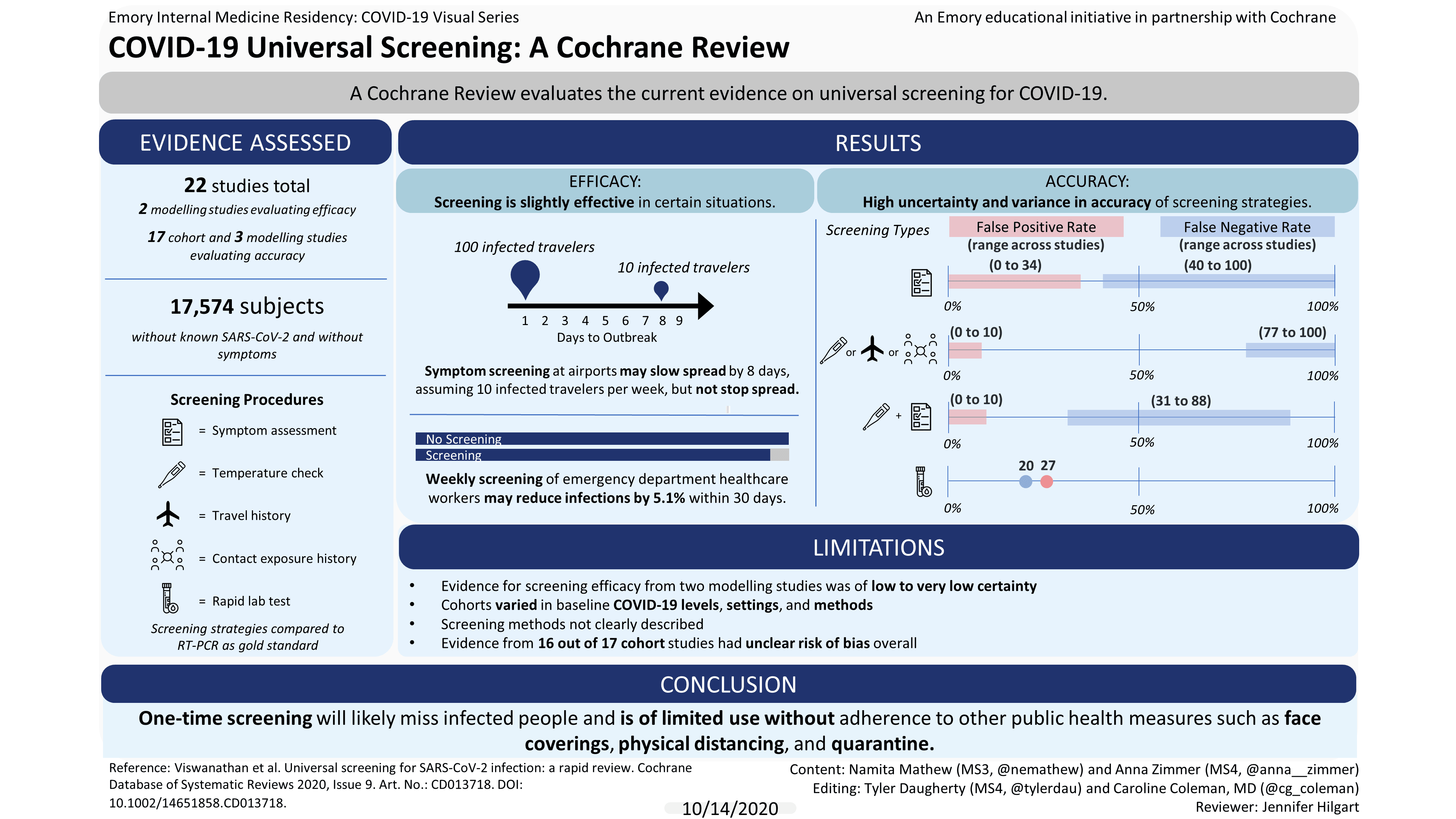
We found 22 studies; 17 assessed people (cohort studies) and five were computer-generated models (modelling studies). Studies took place in USA, Europe, and Asia.
Benefits and negative effects
Two modelling studies reported on the benefits and negative effects of screening. One suggested that asking about symptoms at airports may slightly slow, but not stop, the importation of infected people.
Another model reported that weekly or biweekly screening of healthcare workers may reduce transmission to patients and other healthcare workers in emergency departments.
No studies reported on negative effects of screening.
Identification of infected people
Seventeen cohort studies and three modelling studies reported on whether screening can correctly identify those with and without the virus. Studies varied widely in the baseline level of COVID-19, settings, and methods. All cohort studies compared screening strategies to a ‘gold standard’ test called RT-PCR.
Cohort studies
All screening strategies (17 studies, 17,574 people), incorrectly identified:
· between 20 and 100 out of 100 infected people as healthy;
· between 0 and 38 people out of 100 healthy people as infected
Asking about symptoms (13 studies, 16,762 people ), incorrectly identified:
· between 40 to 100 out of 100 infected people as healthy
· between 0 to 34 out of 100 healthy people as infected
Temperature measurements, asking about international travel, exposure to known infected people and exposure to known or suspected infected people (6 studies, 14,741 people), incorrectly identified:
· between 77 and 100 out of 100 infected people as healthy
· between 0 and 10 out of 100 healthy people as infected
Asking about symptoms plus temperature measurement (2 studies, 779 people), incorrectly identified:
· between 31 and 88 out of 100 infected people as healthy
· between 0 to 10 people out of 100 healthy people as infected
There was insufficient evidence from two small studies on rapid laboratory tests and repeated symptom assessment to tell how accurate they were in identifying healthy and infected people.
Modelling studies
Three studies modelled entry and exit screening in airports. One study missed 70% of infected travellers. Another detected 90% of infections, but used an unrealistic scenario. The third used very unreliable methods so we cannot use evidence from this study.
How confident are we in the results of the studies?
Our confidence in these findings is limited because most studies did not describe their screening methods clearly, some found very few cases of infections and the types of participants and settings varied greatly, making it difficult to judge whether the results apply broadly.
Authors’ conclusions
One-time screening in apparently healthy people is likely to miss people who are infected. We are unsure whether combined screenings, repeated symptom assessment, or rapid laboratory tests are useful.
As more people become infected, screening will identify more cases. However, because screening can miss people who are infected, public health measures such as face coverings, physical distancing, and quarantine for those who are apparently healthy, continue to be very important.
The evidence base for the effectiveness of screening comes from two mathematical modelling studies and is limited by their assumptions. Low-certainty evidence suggests that screening at travel hubs may slightly slow the importation of infected cases. This review highlights the uncertainty and variation in accuracy of screening strategies. A high proportion of infected individuals may be missed and go on to infect others, and some healthy individuals may be falsely identified as positive, requiring confirmatory testing and potentially leading to the unnecessary isolation of these individuals. Further studies need to evaluate the utility of rapid laboratory tests, combined screening, and repeated screening. More research is also needed on reference standards with greater accuracy than RT-PCR.
Given the poor sensitivity of existing approaches, our findings point to the need for greater emphasis on other ways that may prevent transmission such as face coverings, physical distancing, quarantine, and adequate personal protective equipment for frontline workers.
Coronavirus disease 2019 (COVID-19) is caused by the novel betacoronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Most people infected with SARS-CoV-2 have mild disease with unspecific symptoms, but about 5% become critically ill with respiratory failure, septic shock and multiple organ failure. An unknown proportion of infected individuals never experience COVID-19 symptoms although they are infectious, that is, they remain asymptomatic. Those who develop the disease, go through a presymptomatic period during which they are infectious. Universal screening for SARS-CoV-2 infections to detect individuals who are infected before they present clinically, could therefore be an important measure to contain the spread of the disease.
We conducted a rapid review to assess (1) the effectiveness of universal screening for SARS-CoV-2 infection compared with no screening and (2) the accuracy of universal screening in people who have not presented to clinical care for symptoms of COVID-19.
An information specialist searched Ovid MEDLINE and the Centers for Disease Control (CDC) COVID-19 Research Articles Downloadable Database up to 26 May 2020. We searched Embase.com, the CENTRAL, and the Cochrane Covid-19 Study Register on 14 April 2020. We searched LitCovid to 4 April 2020. The World Health Organization (WHO) provided records from daily searches in Chinese databases and in PubMed up to 15 April 2020. We also searched three model repositories (Covid-Analytics, Models of Infectious Disease Agent Study [MIDAS], and Society for Medical Decision Making) on 8 April 2020.
Trials, observational studies, or mathematical modelling studies assessing screening effectiveness or screening accuracy among general populations in which the prevalence of SARS-CoV2 is unknown.
After pilot testing review forms, one review author screened titles and abstracts. Two review authors independently screened the full text of studies and resolved any disagreements by discussion with a third review author. Abstracts excluded by a first review author were dually reviewed by a second review author prior to exclusion. One review author independently extracted data, which was checked by a second review author for completeness and accuracy. Two review authors independently rated the quality of included studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool for diagnostic accuracy studies and a modified form designed originally for economic evaluations for modelling studies. We resolved differences by consensus. We synthesized the evidence in narrative and tabular formats. We rated the certainty of evidence for days to outbreak, transmission, cases missed and detected, diagnostic accuracy (i.e. true positives, false positives, true negatives, false negatives) using the GRADE approach.
We included 22 publications. Two modelling studies reported on effectiveness of universal screening. Twenty studies (17 cohort studies and 3 modelling studies) reported on screening test accuracy.
Effectiveness of screening
We included two modelling studies. One study suggests that symptom screening at travel hubs, such as airports, may slightly slow but not stop the importation of infected cases (assuming 10 or 100 infected travellers per week reduced the delay in a local outbreak to 8 days or 1 day, respectively). We assessed risk of bias as minor or no concerns, and certainty of evidence was low, downgraded for very serious indirectness. The second modelling study provides very low-certainty evidence that screening of healthcare workers in emergency departments using laboratory tests may reduce transmission to patients and other healthcare workers (assuming a transmission constant of 1.2 new infections per 10,000 people, weekly screening reduced infections by 5.1% within 30 days). The certainty of evidence was very low, downgraded for high risk of bias (major concerns) and indirectness. No modelling studies reported on harms of screening.
Screening test accuracy
All 17 cohort studies compared an index screening strategy to a reference reverse transcriptase polymerase chain reaction (RT-PCR) test. All but one study reported on the accuracy of single point-in-time screening and varied widely in prevalence of SARS-CoV-2, settings, and methods of measurement.
We assessed the overall risk of bias as unclear in 16 out of 17 studies, mainly due to limited information on the index test and reference standard. We rated one study as being at high risk of bias due to the inclusion of two separate populations with likely different prevalences. For several screening strategies, the estimates of sensitivity came from small samples.
For single point-in-time strategies, for symptom assessment, the sensitivity from 12 cohorts (524 people) ranged from 0.00 to 0.60 (very low-certainty evidence) and the specificity from 12 cohorts (16,165 people) ranged from 0.66 to 1.00 (low-certainty evidence). For screening using direct temperature measurement (3 cohorts, 822 people), international travel history (2 cohorts, 13,080 people), or exposure to known infected people (3 cohorts, 13,205 people) or suspected infected people (2 cohorts, 954 people), sensitivity ranged from 0.00 to 0.23 (very low- to low-certainty evidence) and specificity ranged from 0.90 to 1.00 (low- to moderate-certainty evidence). For symptom assessment plus direct temperature measurement (2 cohorts, 779 people), sensitivity ranged from 0.12 to 0.69 (very low-certainty evidence) and specificity from 0.90 to 1.00 (low-certainty evidence). For rapid PCR test (1 cohort, 21 people), sensitivity was 0.80 (95% confidence interval (CI) 0.44 to 0.96; very low-certainty evidence) and specificity was 0.73 (95% CI 0.39 to 0.94; very low-certainty evidence). One cohort (76 people) reported on repeated screening with symptom assessment and demonstrates a sensitivity of 0.44 (95% CI 0.29 to 0.59; very low-certainty evidence) and specificity of 0.62 (95% CI 0.42 to 0.79; low-certainty evidence).
Three modelling studies evaluated the accuracy of screening at airports. The main outcomes measured were cases missed or detected by entry or exit screening, or both, at airports. One study suggests very low sensitivity at 0.30 (95% CI 0.1 to 0.53), missing 70% of infected travellers. Another study described an unrealistic scenario to achieve a 90% detection rate, requiring 0% asymptomatic infections. The final study provides very uncertain evidence due to low methodological quality.